Dr. Nako Nakatsuka
Hiring PhD students in Translational Aptamer Biosensing Technologies
Nako Nakatsuka, PhD
Postdoc/principle investigator, Sep. 2018 to Dec. 2023
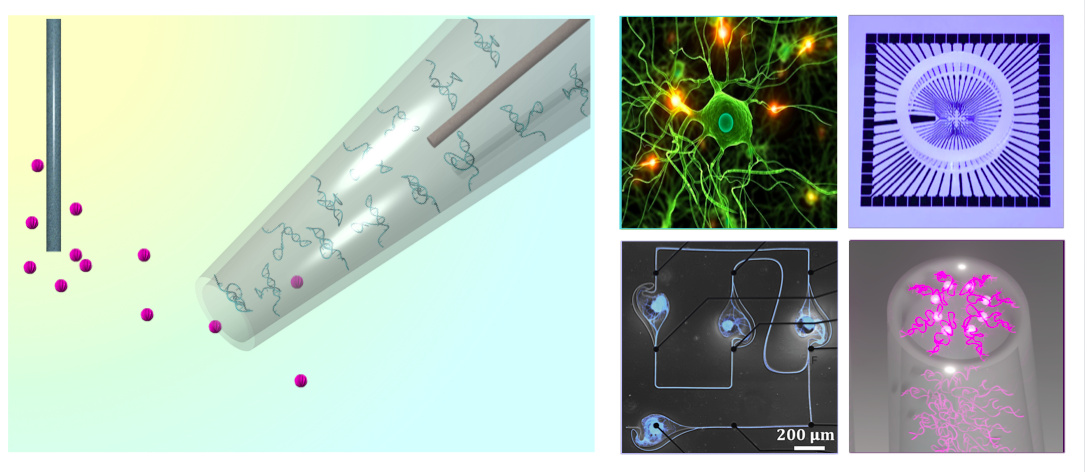
Currently seeking motivated students from multidisciplinary backgrounds such as chemistry, materials science, nanoscience, electrical/mechanical/biomedical engineering, and neuroscience. Thesis opportunities for DNA aptamer-based next-generation biosensors that function in real clinically relevant environments.
Schweiz
Biography
Nako Nakatsuka was raised in Tokyo, Japan and moved to the United States of America to attend Fordham University in New York and pursued a B.S. degree in Chemistry. In 2010, she joined the bionanotechnology research lab of Professor Ipsita A. Banerjee in the Department of Chemistry at Fordham. Her undergraduate research focused on developing peptide-based nanostructures for tissue engineering and drug delivery applications. She was a member of the Division I Fordham cross country and track and field team.
For her Ph.D. in Chemistry, she attended the University of California, Los Angeles and worked in the labs of Professors Anne M. Andrews and Paul S. Weiss. Her thesis research initially focused on the design and development of external pagescreening substratescall_made for the discovery of novel DNA-based molecular recognition elements called aptamers that recognize small-molecules. Neurotransmitter-specific aptamers discovered through collaboration, were integrated into field-effect transistor platforms for electronic neurosensing. Her expertise in external pagesurface functionalizationcall_made and external pagemolecular self-assemblycall_made, external pagechemical patterning strategiescall_made, and external pagebiomolecule target-receptor interactionscall_made, culminated in the design and fabrication of external pageaptamer-based electronic biosensorscall_made that function in physiological environments (e.g. blood and brain fluid). In parallel, she designed and fabricated external pageserotonin-based nanoparticlescall_made as a class of previously unexplored multifunctional nanoplatforms for cancer therapeutics. During her Ph.D., she was a member of the UCLA Triathlon Team and illustrated a external pagechildren's chemistry bookcall_made.
In 2018, Nako received the ETH postdoctoral fellowship and joined the Laboratory of Biosensors and Bioelectronics. Currently as a senior scientist, her research is focused on understanding the mechanisms of neurodegenerative diseases by integrating neurotransmitter-specific DNA aptamers to electrophysiology platforms for simultaneous chemical and electrical recordings. These multifunctional sensors can be interfaced with biological systems such as live neuronal networks and brain tissue ex vivo.
Honors and Awards:
2023: external pageACS Nano Lectureship Awardcall_made
2022: iCanX Young Scientist Award
2021: external pageMIT Technology Review Innovator Under 35call_made
2021: European Biosensors Symposium Best Oral Presentation Prize
2019: Open Innovation in Life Sciences Conference Science Pitch 1st Place Winner
2018: Norma Stoddart Prize for Academic Excellence and Outstanding Citizenship
2018: ETH Postdoctoral Fellowship
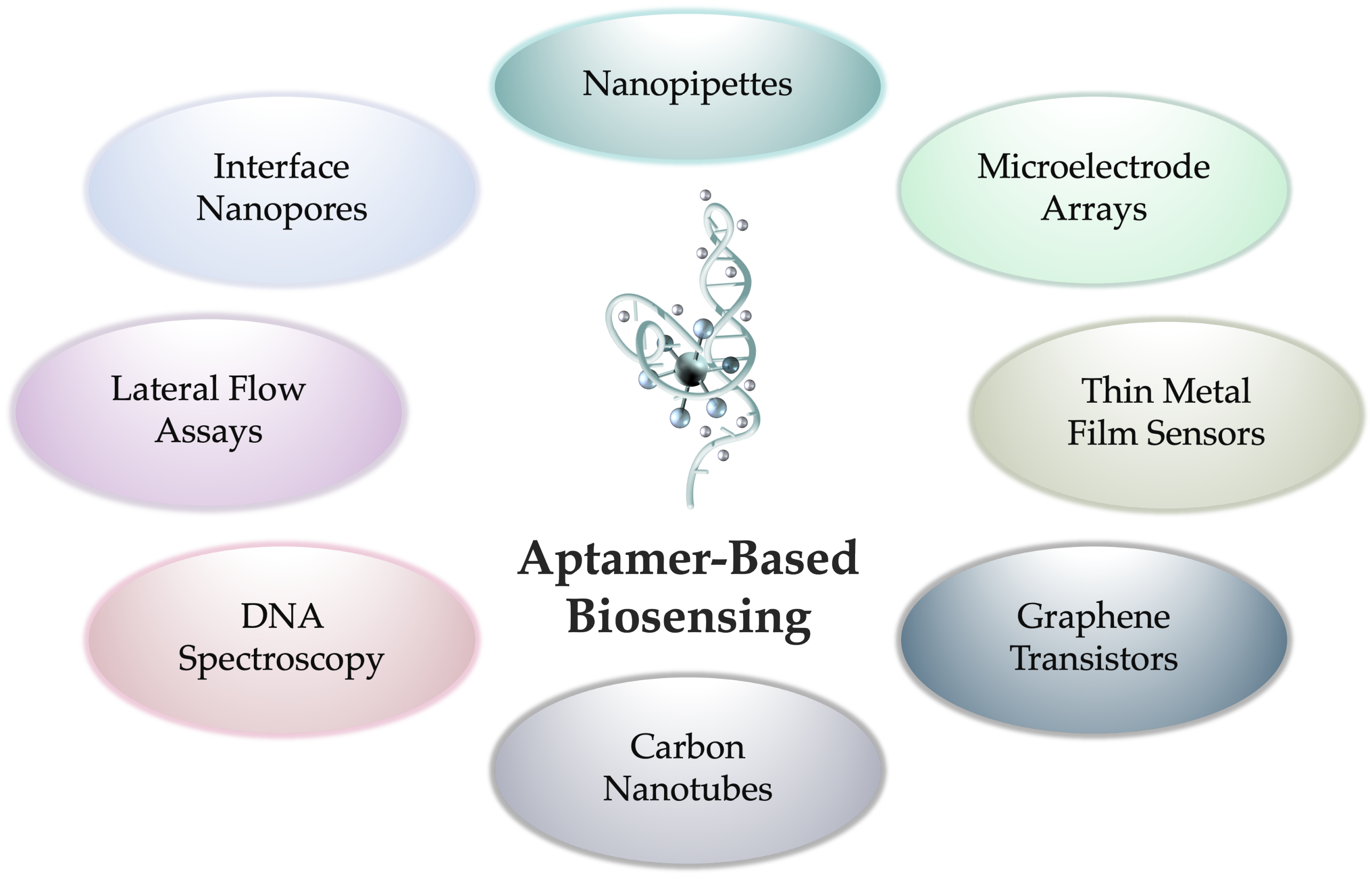
Active Projects:
- Aptamer-modified nanopipettes for neurochemical sensing in complex media
- Aptamer-modified microelectrode arrays to sense neurochemical signaling
- Investigations on DNA aptamer conformational dynamics and kinetics
- Visualization of 3-D intermolecular interactions of aptamer-target complexes
- Single-molecule peptide sequencing via aptamer-modified interface nanopores
- Navigation of neuronal networks on the surface of CMOS-microelectrode arrays
- Field-effect-based aptamer biosensing platforms for point-of-care diagnostics
- Neurochemical aptamer functionalization to graphene field-effect transistors
Research Presentations:
external pageiCanX Young Scientist Award Symposiumcall_made
external pageImperial College London Department of Bioengineering Seminarcall_made
external pageScanlon Group Electrochemistry Webinar Seriescall_made
Publications
2023
1. Stuber A, Douaki A, Hengsteler J, Buckingham D, Momotenko D, Garoli D, Nakatsuka N. Aptamer conformational dynamics modulate neurotransmitter sensing in nanopores, ACS Nano, DOI: 10.1101/2023.03.10.532011 (2023).
2. Shkodra B, Petrelli M, Yang KA, Lugli P, Petti L, Nakatsuka N. Polymeric integration of structure-switching aptmers on transistors for histamine sensing, Faraday Discussions, DOI: 10.1039/D3FD00123G
2022
3. Weaver S, Mohammadi MH, Nakatsuka N. Aptamer-functionalized capacitive biosensors, Biosensors & Bioelectronics, 224: 115014 (2022).
4. Nakatsuka N. Aptamer-field-effect transistors for small-molecule sensing in complex environments, Aptamer Technology Edition, Methods in Molecular Biology, 2570: 187-196 (2022).
2021
5. Moraldo C, Vuille-dit-Bille E, Shkodra B, Kloter T, and Nakatsuka N. Aptamer-Modified Biosensors to Visualize Neurotransmitter Flux, Journal of Neuroscience Methods, 365: 1-13 (2021).
6. Zhao C, Cheung KM, Huang I, Yang H, Nakatsuka N, Liu W, Cao Y, Man T, Weiss PS, Monbouquette HG, Andrews AM. Implantable aptamer field-effect transistor neuroprobes for in vivo neurotransmitter monitoring, Science Advances, 7: 1-10 (2021).
7. Shkodra B, Petrelli M, Angeli MAC, Garoli D, Nakatsuka N, Lugli P, Petti L. Electrolyte-gated carbon nanotube field-effect transistor biosensors: principle and applications, Applied Physics Letters, 8: 041325 (2021).
8. Frutiger A, Tanno A, Hwu S, Tiefenauer R, Vörös J, and Nakatsuka N. Nonspecific Binding - Fundamental Concepts and Consequences for Biosensing Applications, Chemical Reviews, 121(13):8095-8160 (2021).
9. Anthony M, Hordijk I, Nakatsuka N. Justice, equity, diversity, and inclusion seminars: what they do and do not do, ETH Learning Teaching Journal, 3: 65-70 (2021).
10. Fracassi A, Ray A, Nakatsuka N, Passiu C, Tanriver M, Schauenburg D, Scherrer S, Ouald AC, Mandal J, Ramakrishna S, Bode J, Spencer N, Rossi A, and Yamakoshi Y. KAT Ligation for Rapid and Facile Covalent Attachment of Biomolecules to Surfaces, ACS Applied Materials & Interfaces, 13(24):29113-29121 (2021).
11. Nakatsuka N, Heard KJ, Faillétaz A, Momotenko D, Vörös J, Gage FH, and Vadodaria KC. Sensing Serotonin Secreted from Human Serotonergic Neurons using Aptamer-Modified Nanopipettes, Molecular Psychiatry, DOI: s41380-021-01066-5 (2021).
12. Nakatsuka N, Faillétaz A, Eggemann D, Forró C, Vörös J, and Momotenko D. Aptamer Conformational Change Enables Serotonin Biosensing with Nanopipettes, ACS Analytical Chemistry, 93(8):4033-4041 (2021).
13. Nakatsuka N, Abendroth JM, Yang KA, and Andrews AM. Divalent Cation Dependence Enhances Dopamine Aptamer Biosensing, ACS Applied Materials & Interfaces, 13(8):9425-9435 (2021).
2020
14. Cheung KM, Abendroth JM, Nakatsuka N, Zhu B, Yang Y, Andrews AM, and Weiss PS. Detecting DNA and RNA and Differentiating Single-Nucleotide Variations via Field-Effect Transistors, Nano Letters, 20(8):5982-5990 (2020).
2019
15. Cheung KM, Yang KA, Nakatsuka N, Zhao C, Ye M, Jung M, Yang H, Weiss PS, Stojanović MN, and Andrews AM. Phenylalanine Monitoring via Aptamer-Field-Effect Transistor Sensors, ACS Sensors, 4(12):3308-3317 (2019).
16. Hasani-Sadrabadi MM, Sarrion P, Nakatsuka N, Young TD, Taghdiri N, Ansari S, Aghaloo T, Li S, Khademhosseini A, Weiss PS, Moshaverinia A. Hierarchically Patterned Polydopamine-Containing Membranes for Periodontal Tissue Engineering, ACS Nano, 13(4):3830-3838 (2019).
2018
17. Nakatsuka N, Yang KA, Abendroth JM, Cheung KM, Xu X, Yang HY, Zhao C, Zhu B, Rim YS, Yang Y, Weiss PS, Stojanović MN, and Andrews AM. Aptamer Field-Effect Transistors Overcome Debye Length Limitations for Small-Molecule Sensing, Science, 362:319-324 (2018).
18. Nakatsuka N, Cao HH, Deshayes S, Melkonian AL, Kasko AM, Weiss PS, and Andrews AM. Aptamer Recognition on Multiplexed Small-Molecule-Functionalized Substrates, ACS Applied Materials & Interfaces, 10(28):23490-23500 (2018).
19. Nakatsuka N, Hasani-Sadrabadi MM, Cheung KM, Young TD, Bahlakeh G, Moshaverinia A, Weiss, PS, Andrews AM. Polyserotonin Nanoparticles as Multifunctional Materials for Biomedical Applications, ACS Nano, 12(5):4761-4774 (2018).
20. Cao HH*, Nakatsuka N*, Deshayes S, Abendroth JM, Yang H, Weiss PS, Kasko AM, and Andrews AM. Small-Molecule Patterning via Pre-Functionalized Alkanethiols, Chemistry of Materials, 30(12):4017-4030 (2018) *authors contributed equally.
2017
21. Nakatsuka N and Andrews AM. Differentiating Siblings: The Case of Dopamine and Norepinephrine, ACS Chemical Neuroscience, 8:218-220 (2017).
22. Yang KA, Chun HS, Zhang Y, Pecic S, Nakatsuka N, Andrews AM, Worgall TP, Stojanović MN. High-Affinity Nucleic-Acid Based Receptors for Steroids, ACS Chemical Biology, 12(12):3103-3112 (2017).
23. Cao HH, Nakatsuka N, Liao WS, Serino A, Cheunkar S, Yang H, Weiss PS, and Andrews AM. Advancing Biocapture Substrates via Chemical Lift-Off Lithography, Chemistry of Materials, 29(16):6829-6839 (2017).
24. Abendroth JM, Nakatsuka N, Ye M, Kim D, Fullerton EE, Andrews AM, and Weiss PS. Analyzing Spin Selectivity in DNA-Mediated Charge Transfer via Fluorescence Microscopy, ACS Nano, 11(7): 7516-7526 (2017).
2016
25. Nakatsuka N and Andrews AM. Nanoscale Neurochips to Enable High Resolution in Vivo Neurotransmitter Sensing, Neuropsychopharmacology. 41:378-379 (2016).
2015
26. Cao HH, Nakatsuka N, Serino AC, Liao WS, Cheunkar S, Yang H, Weiss PS, and Andrews AM. Controlled DNA Patterning by Chemical Lift-Off Lithography: Matrix Matters, ACS Nano, 9(11):11439-11454 (2015).
27. Kim J, Rim YS, Chen H, Cao HH, Nakatsuka N, Hinton HL, Zhao C, Andrews AM, Yang Y, and Weiss PS. Fabrication of High-Performance Ultrathin In2O3 Film Field-Effect Transistors and Biosensors Using Chemical Lift-Off Lithography, ACS Nano, 9(4):4572-4582 (2015).
2011 - 2013
28. Nakatsuka N, Barnaby SN, Fath KR, Tsiola A, Williams BA, and Banerjee IA. Self-Assembling Peptide Assemblies Bound to ZnS Nanoparticles and their Interactions with Mammalian Cells, Colloids and Surfaces B: Biointerfaces, 103:405-415 (2013).
29. Barnaby SN, Nakatsuka N, Frayne SH, Fath KR, and Banerjee IA. Formation of Hyaluronic Acid Ellagic Acid Microfiber Hybrid Hydrogels and their Applications, Colloid Polymer Science, 291(3):515-525 (2013).
30. Sarker NH, Barnaby SN, Dowdell AP, Nakatsuka N, and Banerjee IA. Biomimetic Formation of Pd and Au-Pd Nanocomposites and their Catalytic Applications, Soft Materials, 11(4):403-413 (2013).
31. Frayne SH, Barnaby SN, Nakatsuka N, and Banerjee IA. Growth and Properties of CdSe Nanoparticles on Ellagic Acid Biotemplates for Photodegredation Applications, Materials Express, 2(4):335-343 (2012).
32. Barnaby SN, Fath KR, Nakatsuka N, Sarker NH, and Banerjee IA. Formation of Calcium Phosphate Ellagic Acid Composites by Layer by Layer Assembly for Cellular Attachment to Osteoblasts, Journal of Biomimetics, Biomaterials, and Tissue Engineering, 13(1):1-17 (2012).
33. Sarker NH, Barnaby SN, Frayne SH, Nakatsuka N, and Banerjee IA. Biomimetic Growth of ZnO-Gallic Acid Nanohybrid Assemblies and their Applications, Journal of Nanoparticle Research, 14(4):1-12 (2012).
34. Nakatsuka N, Barnaby SN, Fath KR, and Banerjee IA. Fabrication of Collagen-Elastin Bound Peptide Microtubes for Mammalian Cell Attachment, Journal of Biomaterials Science, Polymer Edition, 22(18): 1843-1862 (2011).
35. Nakatsuka N, Barnaby SN, and Banerjee IA. From Self-Assembly to Nanoelectronics, Sensors and Medicine - A Biological Approach to the Growth and Applications of Nanowires – A review, Chapter I, in book entitled: Nanowires, Properties, Synthesis and Applications), (ISBN #978-1-61470-287-0) Nova Science Publishers (2011).
